In an evolving health landscape, emerging research continues to highlight concerns that could impact everyday wellbeing. Here’s the key update you should know about:
Widely used weight-loss and diabetes drugs show clear benefits, but emerging evidence clarifies their side effects, rare risks, and what clinicians should monitor long term.
A recent review article published in the Journal of Clinical Investigation summarized the adverse effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) and dual incretin receptor agonists such as tirzepatide.
Of the two incretin hormones, glucagon-like peptide-1 (GLP-1) has therapeutic potential to reduce body weight and plasma glucose levels. The other incretin hormone, glucose-dependent insulinotropic polypeptide (GIP), generally shows impaired insulinotropic activity and limited glucose-lowering effectiveness in type 2 diabetes (T2D). As such, GLP-1 has become the parent compound for the development of GLP-1RAs.
GLP-1 acts through the GLP-1 receptor (GLP-1R), primarily expressed in the brain, pancreatic islets, and gastrointestinal (GI) tract. GLP-1RAs are now widely used and provide beneficial GLP-1R-mediated effects on glycemic control and body weight. However, GLP-1R stimulation may induce adverse responses. In this review, researchers summarized the adverse effects associated with GLP-1RA treatment.
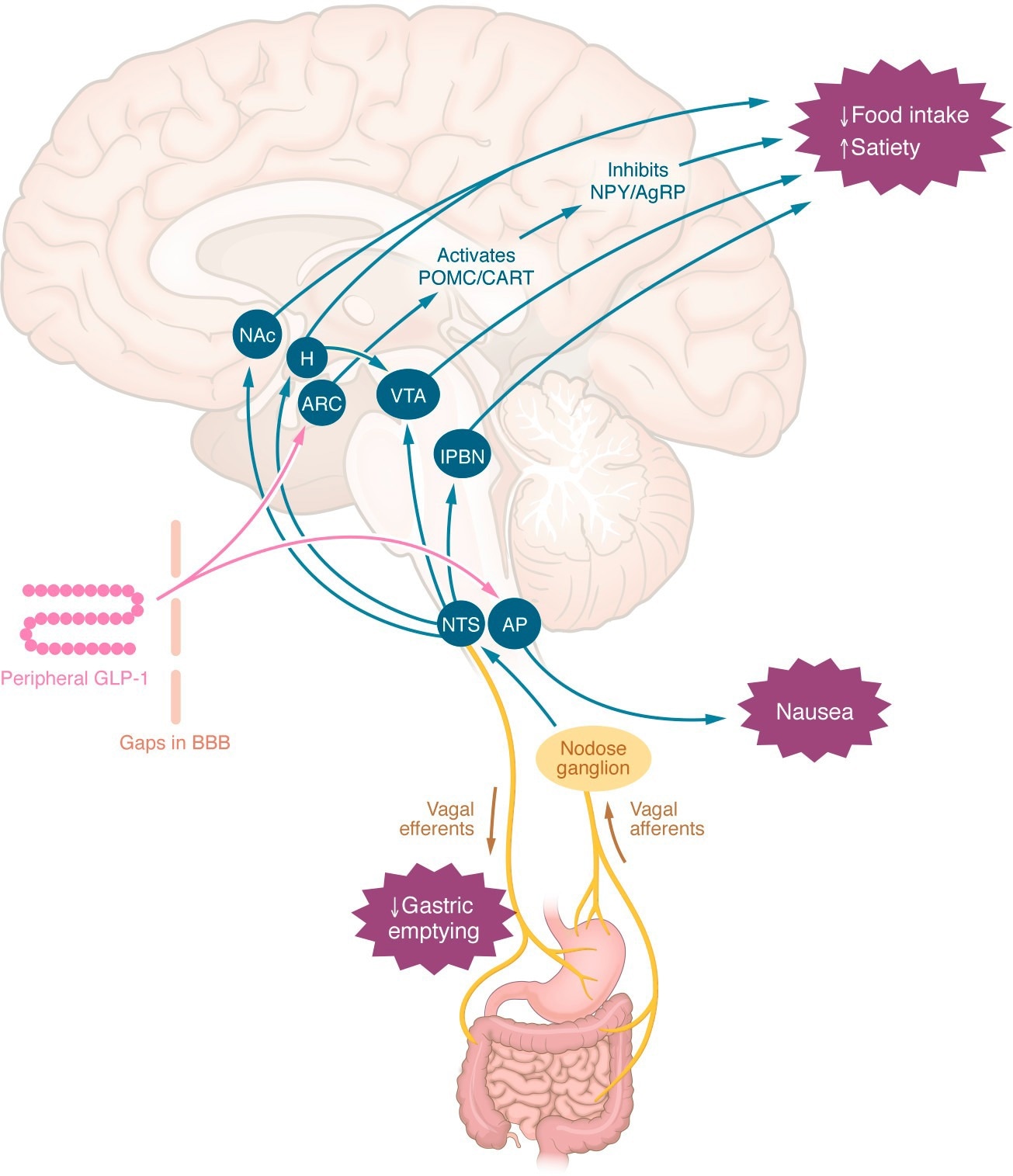
Central mechanisms by which peripheral GLP-1RAs may reduce appetite or induce nausea. Peripheral GLP-1 or GLP-1RAs may interact with appetite-regulating regions of the brain via gaps in the blood-brain barrier, via tanycyte uptake, or indirectly via the nodose ganglia. The effects to slow gastric emptying, induce nausea, and reduce energy intake are independent. AgRP, agouti-related peptide; AP, area postrema; ARC, arcuate nucleus; BBB, blood-brain barrier; CART, cocaine- and amphetamine-regulated transcript; H, hypothalamus; lPBN, lateral parabrachial nucleus; NAc, nucleus accumbens; NPY, neuropeptide Y; NTS, nucleus tractus solitarius; POMC, proopiomelanocortin; VTA, ventral tegmental area.
Gastrointestinal and Pancreatic Safety Concerns
Gastrointestinal adverse effects are the most commonly reported side effects of GLP-1RAs. A systematic review of 39 randomized controlled trials (RCTs) found increased risks of vomiting, nausea, constipation, and diarrhea with GLP-1RAs in non-diabetic individuals compared to placebo. Another review of 38 RCTs involving T2D patients observed nausea in 19% of participants treated with GLP-1RAs and vomiting in 7.6%. In a phase II trial of subcutaneous semaglutide, rapid dose escalation led to greater weight loss but more adverse events.
Tirzepatide is a co-agonist of the GIP receptor and GLP-1R and demonstrates greater efficacy for weight loss and glucose lowering than selective GLP-1RAs, with a broadly similar adverse event profile. In animal experiments, GLP-1RAs induce frequent vomiting, whereas GIP receptor agonists do not. In one clinical trial, 5 mg per week tirzepatide was slightly more effective than 1 mg per week semaglutide in reducing body weight and glycated hemoglobin (HbA1c).
Adverse GI effects were only marginally less prevalent with tirzepatide. However, a systematic review reported that tirzepatide conferred the highest risk of vomiting. A large cardiovascular outcomes trial found that higher proportions of tirzepatide recipients reported vomiting, diarrhea, and nausea compared to those taking a selective GLP-1RA. These findings argue against the notion that GIP co-agonism reduces the risk of adverse GI events.
Delayed gastric emptying associated with GLP-1RAs may increase the volume of retained gastric contents before endoscopic or surgical procedures, although evidence directly linking this to aspiration pneumonia remains limited and sometimes conflicting. Some analyses also suggest an increased risk of biliary disease, particularly cholelithiasis, with GLP-1RA therapy.
Earlier concerns regarding acute pancreatitis and pancreatic cancer have largely been alleviated by long-term randomized trials that have not confirmed a causal association, although reporting biases and diagnostic complexity remain considerations, and continued pharmacovigilance is recommended.
Thyroid Cancer Risk and C-Cell Findings
Concerns about medullary thyroid carcinoma emerged from rodent studies showing increased calcitonin secretion and C-cell growth after liraglutide treatment. While GLP-1R expression has been demonstrated in rodent thyroid C cells, the receptor has generally not been detected in healthy human or primate thyroid C cells, or has been detected only in small subsets.
In contrast, many hyperplastic C cells and medullary thyroid carcinomas in humans express GLP-1R. Data from France suggest a higher risk of medullary thyroid carcinoma in people treated with GLP-1RAs compared to other glucose-lowering agents. A meta-analysis also reported diagnoses of medullary thyroid carcinoma in patients taking GLP-1RAs, reinforcing the at-risk status as a contraindication to GLP-1RA treatment. However, absolute event numbers remain low, and epidemiological findings for other thyroid cancer subtypes are inconsistent, with several studies reporting no increased risk.
Ocular and Psychiatric Safety Signals
Retinopathy and neuropsychiatric outcomes have generated additional safety discussions. In a cardiovascular outcomes trial, semaglutide treatment increased retinopathy complications. A retrospective analysis suggested that these complications were primarily observed among participants with proliferative or preproliferative retinopathy at baseline who experienced substantial reductions in HbA1c. However, baseline retinopathy was not systematically assessed, and other trials excluding pre-existing retinopathy or maculopathy have reported comparable event rates between treatment groups. One cohort study reported a higher risk of non-arteritic anterior ischemic optic neuropathy (NAION) in individuals prescribed semaglutide.
A large multicenter database study confirmed this association; however, the incidence was 14.5 per 100,000 person-years, and the increased risk attributable to semaglutide was modest. A five-year longitudinal cohort study reported that semaglutide exposure doubled the risk of NAION, whereas another study found no increased risk. Further research is needed to clarify causality.
Obesity and T2D are risk factors for depression and suicidal ideation. Metabolic surgery, which increases endogenous GLP-1 secretion, has been associated with a higher risk of self-harm and suicide. A large retrospective study found a two-fold increased risk of anxiety and suicidal behavior and a three-fold increased risk of major depression among GLP-1RA users.
Conversely, some studies report decreased depression risk with GLP-1RA use and suggest potential antidepressant effects. Two recent meta-analyses found no association between GLP-1RAs and suicidal ideation. A systematic review reported no associations between GLP-1RAs and serious psychiatric effects, including psychosis, major depression, or suicide. However, heterogeneity in outcome definitions and reporting complicates interpretation.
Risk–Benefit Assessment and Pharmacovigilance Needs
Given the widespread use of GLP-1RAs and other incretin-based agents for obesity and T2D, comprehensive evaluation of even common GI adverse effects remains limited. Improved pharmacovigilance and standardized adverse event reporting would enhance understanding of risk-benefit profiles for individual GLP-1RAs and indications. Particular attention is warranted for diverse populations, including older adults, individuals with advanced renal disease, pregnant patients, and those at risk of lean mass loss during rapid weight reduction.
Journal reference:
- Jalleh RJ, Talley NJ, Horowitz M, Nauck MA (2026). The science of safety: adverse effects of GLP-1 receptor agonists as glucose-lowering and obesity medications. Journal of Clinical Investigation, 136(4). DOI: 10.1172/JCI194740, https://www.jci.org/articles/view/194740

