Chemists Grow Diamonds With an Electron Beam

Synthetic diamond research examines organic molecular interactions under the microscope.
Scientists have long developed different techniques to produce artificial diamonds, but a new method from researchers, including a team at the University of Tokyo, offers surprising advantages. By preparing samples in a specific way before exposing them to an electron beam, the group discovered that their process not only supports diamond formation but also shields organic materials from the damage that such beams typically cause. This breakthrough could open the door to more advanced imaging and analysis methods.
Traditionally, diamond creation relies on transforming carbon sources under extreme physical conditions. These include pressures of tens of gigapascals and temperatures reaching thousands of kelvin, where diamond remains thermodynamically stable. Another approach involves chemical vapor deposition, a method where diamond is actually unstable. In contrast, Professor Eiichi Nakamura and his colleagues at the University of Tokyo’s Department of Chemistry pursued a low-pressure strategy that makes use of carefully controlled electron irradiation applied to a carbon cage molecule known as adamantane (C10H16).
What makes adamantane particularly promising is its structural similarity to diamond. Both share a tetrahedral, symmetrical carbon framework with atoms arranged in the same spatial configuration. This makes adamantane an appealing starting material for nanodiamond production. However, successful conversion depends on the precise removal of adamantane’s C–H bonds to allow new C–C bonds to form, while the individual building blocks assemble into a three-dimensional diamond lattice. Although this requirement was already known in the field,
“The real problem was that no one thought it feasible,” said Nakamura.
From Theory to Observation
Previously, mass spectrometry, an analytical technique that sorts ions according to their differing mass and charge, had shown that single-electron ionization could be used to facilitate such C–H bond cleavage. Mass spectrometry, however, can only infer structure formation in the gas phase, and is unable to isolate products from intermolecular reactions.
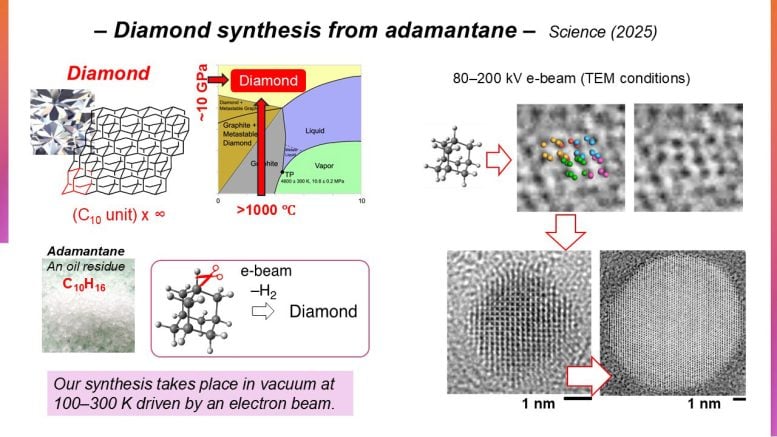
The team was prompted to monitor electron-impact ionization of solid adamantane at atomic resolution using an analytical and imaging technique called transmission electron microscopy (TEM), irradiating submicrocrystals at 80-200 kiloelectron volts at 100-296 kelvins in vacuum for tens of seconds. Not only would the method reveal the evolution of the polymerized nanodiamond formation, but provide powerful ramifications for the potential of TEM as a tool to resolve the controlled reactions of other organic molecules.
For Nakamura, who had worked on synthetic chemistry for 30 years and computational quantum chemical calculations for 15 years, the study offered a breakthrough opportunity. “Computational data gives you ‘virtual’ reaction paths, but I wanted to see it with my eyes,” he said. “However, the common wisdom among TEM specialists was that organic molecules decompose quickly as you shine an electron beam on them. My research since 2004 has been a constant battle to show otherwise.”
The Birth of Nanodiamonds
The process yielded defect-free nanodiamonds of cubic crystal structure, accompanied by hydrogen gas eruptions, up to 10 nanometers in diameter under prolonged irradiation. The time-resolved TEM images illustrated the passage of formed adamantane oligomers transforming into spherical nanodiamonds, moderated by the C–H cleavage rate. The team also tested other hydrocarbons, which failed to form nanodiamonds, highlighting the suitability of adamantane as a precursor.
The findings open a new paradigm for understanding and controlling chemistry in the fields of electron lithography, surface engineering, and electron microscopy. Analysis of the nanodiamond conversion supports long-standing ideas that diamond formation in extraterrestrial meteorites and uranium-bearing carbonaceous sedimentary rocks may be driven by high-energy particle irradiation. Nakamura also pointed to the basis it provides for synthesizing doped quantum dots, essential to the construction of quantum computers and sensors.
As the latest chapter in a 20-year-long research dream, Nakamura said, “This example of diamond synthesis is the ultimate demonstration that electrons do not destroy organic molecules but let them undergo well-defined chemical reactions, if we install suitable properties in molecules to be irradiated.” By forever changing the game in fields employing electron beams for research, his dream could now provide the vision for scientists to uncloud interactions under electron irradiation.
Reference: “Rapid, low-temperature nanodiamond formation by electron-beam activation of adamantane C–H bonds” by Jiarui Fu, Takayuki Nakamuro and Eiichi Nakamura, 4 September 2025, Science.
DOI: 10.1126/science.adw2025
Funding: JSPS KAKENHI, JST PRESTO
Never miss a breakthrough: Join the SciTechDaily newsletter.
Source link

