Study design
This study was a prospective decentralized randomized controlled trial with technology-enabled, remote recruitment, care delivery, and data collection conducted in Germany. The study’s primary endpoint was defined as the change in body weight assessed at 6 months of intervention. The study had a crossover design with participants in the intervention group continuing to receive the intervention for an additional 6 months (after primary endpoint assessment), whilst participants in the control group waited to receive the intervention for 6 months and then received the intervention for 6 months. Further analyses are planned for both 48 and 96 weeks. The study protocol has been approved by the ethics committee of the Berlin Medical Association (vote number: Eth-57/23) and was registered in the German Clinical Trials Register (Registration number: DRKS00033045). All participants provided written informed consent before participation, and all procedures were performed in accordance with the relevant guidelines and regulations.
Study population
Participants were recruited via multiple social media platforms (e.g., Facebook, Instagram) and directly via the Oviva website between 27th November 2023 and 7th March 2024. Participants who met the following eligibility criteria were included: adults (females, males, non-binary), age between 18 and 75 years, BMI 30–45 kg/m², with a diagnosis of obesity confirmed by a physician, ownership of a smartphone compatible with the Oviva Direkt app, no severe conditions contraindicating lifestyle changes, and no recent or planned use of weight loss medications. Inclusion criteria were checked through a pre-screening survey and video consultations with a medical investigator and the study team. Informed consent was provided via a digital signing procedure. Included participants were randomized via stratified block randomization into the intervention group or control group in a ratio of 1:1. Stratification aimed to ensure that the influencing variables of sex, age, and BMI were equally distributed in both study groups. Pre-planned recruitment quotas to ensure even distribution of participants across the strata were in place. All participants attended five digital, remote study visits (baseline, week 6, week 12, week 18, week 24) and filled in digital questionnaires at these timepoints. Participants received compensation for completing the study visits (electronic vouchers worth 20 EUR at 3 months and 60 EUR at 6 months).
Digital health application
The digital health application Oviva Direkt for Obesity (Oviva AG, Potsdam, Germany) is an app-based, multimodal weight management intervention (targeting diet, physical activity, and disease-related psychological states and behaviors) in accordance with the German guidelines for the prevention and treatment of obesity [7]. The Oviva Direkt for Obesity app is certified as a medical device class IIa and available for iOS and Android. To ensure participants’ safety and the appropriate use of the medical device, a certified dietitian checked in with the participants shortly after they began the therapy and again after 3 months. During the check-ins, the dietitians addressed potential safety issues and explained the functioning of Oviva Direkt for Obesity to empower participants to self-administer the intervention independently. In addition, the dietitians were available via a reactive private chat.
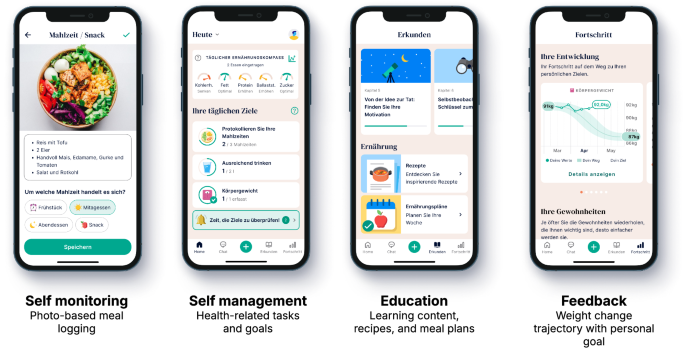
Oviva Direkt for Obesity is a self-administered, app-based therapy designed to support participants to achieve clinically meaningful weight loss. Key intervention elements are self-monitoring, self-management, education, and feedback (details are provided in Table 1, Fig. 1, and in the app manual [8]).
Intervention elements in Oviva Direkt for Obesity.
The mechanism of action incorporates behavior change techniques (BCT) that enable behavioral modifications, which in turn drive weight loss [9]. For example, participants use the photo-based meal logging feature that documents their meals in a nutrition diary (Fig. 1). The act of meal logging corresponds to the BCT self-monitoring, which leads to heightened self-awareness of the types and quantities of food consumed, which supports participants to make conscious, alternative food choices [10]. The generated nutrition diary (based on multiple meal logs) digitally embodies the BCT feedback on behavior, again designed to alter undesirable dietary intake. Importantly, Oviva Direkt for Obesity has been continuously improved based on feedback from patients and health care professionals since the first study on its efficacy [6]. The educational content was improved for readability and actionability. Calorie-reduced, lower-carb meal plans including recipes were integrated, and the goal-setting functionality was upgraded, allowing participants to set and track therapy-related goals (Fig. 1).
Participants in the control group were eligible for care as usual at their discretion, which typically consisted of counseling by a general practitioner, and received access to Oviva Direkt for Obesity at 6 months.
Data collection
Due to the decentralized setup of the study, data were collected remotely during video calls as well as via electronic questionnaires (provided via e-mail and personal links). Sociodemographic data, comorbidities, and concomitant medication were collected at baseline via questionnaires. Study assistants collected weight data via provided, calibrated digital scales in supervised study calls via video calls at all measurement time points (baseline, 6, 12, 18, and 24 weeks). Quality of life and food literacy data were collected via questionnaires at baseline, 12, and 24 weeks. Quality of life was assessed with the WHOQOL-BREF [11], consisting of a score for the four components: physical health, psychological component, social relationships, and the environmental component. Component scores had a range of 0 to 100, while the individual questions were scored on a scale of 1 to 5, with higher values representing a higher quality of life. Also, question 1 (“How do you rate your quality of life?”) and question 11 (“Can you accept your appearance?”) of the WHOQOL-BREF were used to assess the perceived overall quality of life and the acceptance of the physical appearance of the respondent, respectively. Food literacy was assessed with the Short Food Literacy Questionnaire (SFLQ; example question: “If I have questions about healthy eating, I know where I can find information.”) [12]. A higher SFLQ sum score (range of 0–52) indicated higher food literacy. All outcome analyses for the data above were prespecified.
Statistical analyses
The primary endpoint was weight loss expressed as a percent change in body weight at 6 months. The primary analysis population was the intention-to-treat (ITT) population with missing values imputed using multiple imputation by chained equations (MICE) to allow for flexible modeling of missing data with multiple imputations across variable types while preserving relationships [13]. For the primary endpoint weight change, a multiple linear regression analysis adjusted for sex, age, BMI category, baseline weight, comorbidities, and use of concomitant medication (yes/no) was conducted for comparison between the intervention and control group after 6 months. The Bonferroni–Holm procedure was used on each p value for multiple comparisons. Subgroup analyses (unpowered) were conducted across the three strata (sex, age, BMI category; using the ITT population) by fitting the regression model to each stratum of the subgroups. For the secondary endpoint quality of life, the score for each of the four domains of the WHOQOL-BREF (physical health, psychological well-being, social relationships, environment) was calculated, which resulted in scores between 0 (lowest health state) to 100 (perfect state of health) for each domain. For each domain, the difference in scores between baseline and 6 months was calculated, and a multiple linear regression model with the adjustments specified above was conducted, allowing for comparisons between treatment arms. For the exploratory endpoints (relative and absolute weight change over time, change in quality of life over time, change in food literacy over time) mixed effects models for multiple measurements adjusted for sex, age, BMI category, baseline weight were used. Moreover, a logistic regression model was employed to identify the proportion of responders—defined as a decrease in body weight from baseline to 6 months of 5%, 7.5%, or 10%. Sensitivity analyses were conducted to assess the effect of missing data and the imputation mechanism on the calculated group effect using the full analysis set (FAS) population, the complete cases (CC) population, as well as last-observation-carried-forward imputation (LOCF) and copy-increments-in-reference imputation (CIR). Sample size was calculated using a significance level of 0.05, a statistical power of 90%, and an effect size of d = 0.78 (Cohen’s d; estimated from [6]). An assumed 25% drop-out rate, as well as augmentation of the sample to allow for sufficient representation within the strata, yielded a sample size of 160. All statistical analyses were performed using R Version 4.4.2 and RStudio Version 2024.12.0 + 467.
Source link

