Scientists Discover Cancer’s “Power-Up” – and a New Way To Switch It Off
The discovery of a defensive mechanism could help stop cancer before it spreads.
Cancer cells rapidly increase their energy output when physically squeezed, according to a study in Nature Communications. This immediate burst of energy is the first documented defensive response that helps cells repair DNA damage and endure the crowded conditions inside the human body.
The results help explain how cancer cells survive mechanical challenges such as crawling through a tumor microenvironment, slipping into porous blood vessels, or withstanding the forces of the bloodstream. Identifying this mechanism could point to strategies that hold cancer cells in place before they spread.
Researchers at the Centre for Genomic Regulation (CRG) in Barcelona uncovered the effect using a specialized microscope that compresses living cells to about three microns in width, roughly one thirtieth the diameter of a human hair. They observed that, within seconds of compression, mitochondria in HeLa cells moved to the surface of the nucleus and delivered extra ATP, the molecular energy source used by cells.
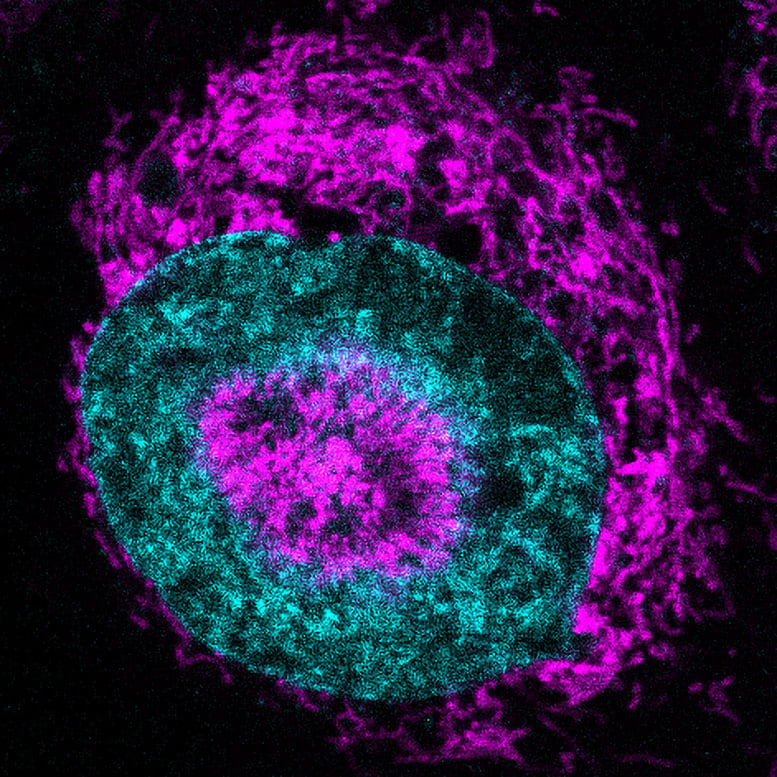
“It forces us to rethink the role of mitochondria in the human body. They aren’t these static batteries powering our cells, but more like agile first responders that can be summoned in emergency situations when cells are literally pressed to the limit,” says Dr. Sara Sdelci, co-corresponding author of the study.
Halo of Mitochondria
The mitochondria formed a halo so tight that the nucleus dimpled inward. The phenomenon was observed in 84 percent of confined HeLa cancer cells, compared with virtually none in floating, uncompressed cells. The researchers refer to the structures “NAMs,” for nucleus-associated mitochondria.
To find out what NAMs did, the researchers deployed a fluorescent sensor that lights up when ATP enters the nucleus. The signal soared by around 60 percent within three seconds of the cells being squeezed. “It’s a clear sign the cells are adapting to the strain and rewiring their metabolism,” says Dr. Fabio Pezzano, co-first author of the study.
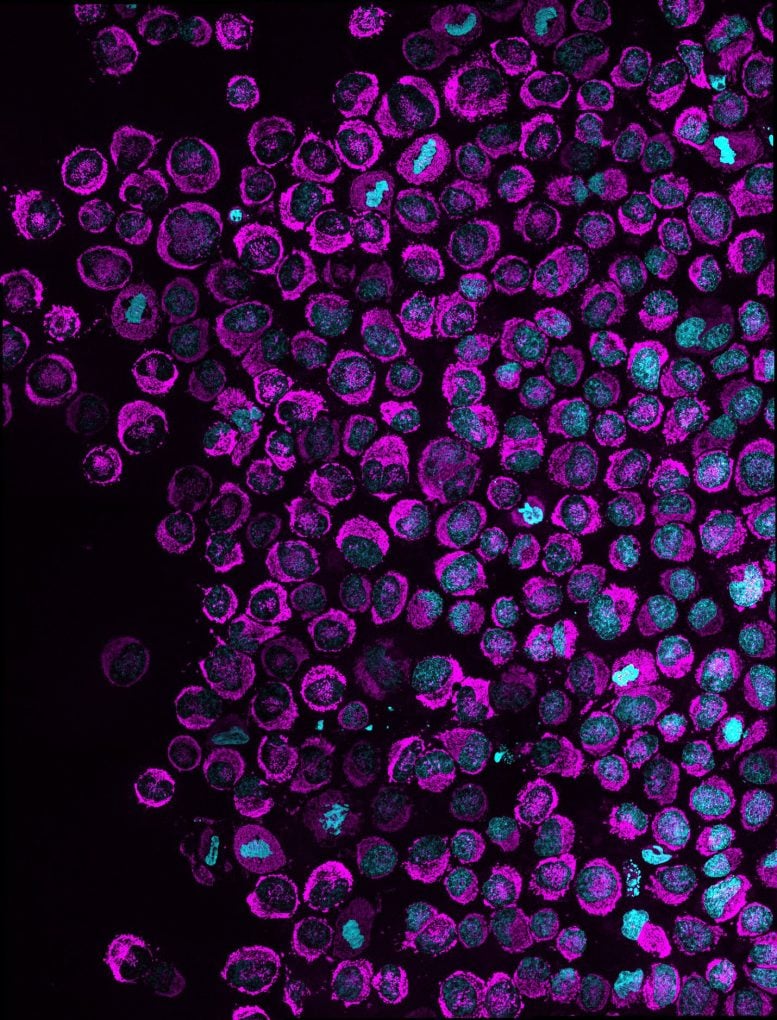
Subsequent experiments revealed why the power surge matters. Mechanical squeezing puts DNA under stress, snapping strands and tangling the human genome. Cells rely on ATP-hungry repair crews to loosen DNA and reach broken sites to mend the damage. Squeezed cells that received the extra boost of ATP repaired DNA within hours, while those without stopped dividing properly.
To confirm relevance for the disease, the researchers also examined breast‑tumor biopsies from 17 patients. The NAM halos appeared in 5.4 percent of nuclei at invasive tumor fronts versus 1.8 percent in the dense tumor core, a three‑fold difference. “Seeing this signature in patient biopsies convinced us of the relevance beyond the lab bench,” explains Dr. Ritobrata (Rito) Ghose, co-first author of the study.
The Cellular Scaffold Behind NAMs
The researchers were also able to study the cellular engineering which makes the mitochondrial rush possible. Actin filaments, the same protein cables that let muscles flex, compound around the nucleus, while the endoplasmic reticulum throws a mesh-like net. The combined scaffold, the study shows, physically traps the NAMs in place, forming the halo-like structure. When the researchers treated cells with latrunculin A, a drug that dismantles actin, NAM formation collapsed, and the ATP tide receded.
If metastatic cells depend on NAM-driven ATP surges, drugs that block the scaffold could make tumors less invasive without broadly poisoning mitochondria and sparing healthy tissues. “Mechanical stress responses are an underexplored vulnerability of cancer cells that can open new therapeutic avenues,” says Dr. Verena Ruprecht, co-corresponding author of the study.
While the study looked at cancer cells, the authors of the study stress that the phenomenon is likely a universal phenomenon in biology. Immune cells squeezing through lymph nodes, neurons extending branches, and embryonic cells during morphogenesis all experience similar physical forces.
“Wherever cells are under pressure, a nuclear energy boost is likely safeguarding the integrity of the genome,” concludes Dr. Sdelci. “It’s a completely new layer of regulation in cell biology, marking a fundamental shift in our understanding of how cells survive intense periods of physical stress.”
Reference: “Mitochondria-derived nuclear ATP surge protects against confinement-induced proliferation defects” by Ritobrata Ghose, Fabio Pezzano, Rémi Badia, Savvas Kourtis, Ilir Sheraj, Shubhamay Das, Antoni Gañez Zapater, Upamanyu Ghose, Sara Musa-Afaneh, Lorena Espinar, Albert Coll-Manzano, Katja Parapatics, Saška Ivanova, Paula Sànchez-Fernàndez-de-Landa, Dragana Radivojevikj, Valeria Venturini, Stefan Wieser, Antonio Zorzano, André C. Müller, Verena Ruprecht and Sara Sdelci, 30 July 2025, Nature Communications.
DOI: 10.1038/s41467-025-61787-x
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google, Discover, and News.
Source link

