This Plant-Inspired Molecule Could Be the Key to Artificial Photosynthesis
Swiss researchers have designed a plant-inspired molecule that mimics photosynthesis and can hold four electric charges when exposed to light.
This ability to store multiple charges could be the key to creating solar fuels such as hydrogen, methanol, or synthetic petrol — fuels that would be carbon-neutral because they release only as much CO2 as was used to produce them.
Harnessing Plant-Inspired Solar Power
Plants capture sunlight and use it to transform carbon dioxide into sugars that store energy. This process, known as photosynthesis, underpins nearly all life on Earth. The sugars produced by plants serve as fuel for animals and humans, who release the stored energy by breaking them down. That process returns carbon dioxide to the atmosphere, completing the natural cycle.
Scientists hope to use this same principle as a guide for developing clean fuels. By copying the way plants convert light, researchers aim to generate energy-rich compounds directly from sunlight. These solar fuels include hydrogen, methanol, and synthetic petrol. When burned, they would release only the same amount of carbon dioxide that was originally required to create them. In effect, the entire process would be carbon-neutral.
A Molecule That Stores Four Charges
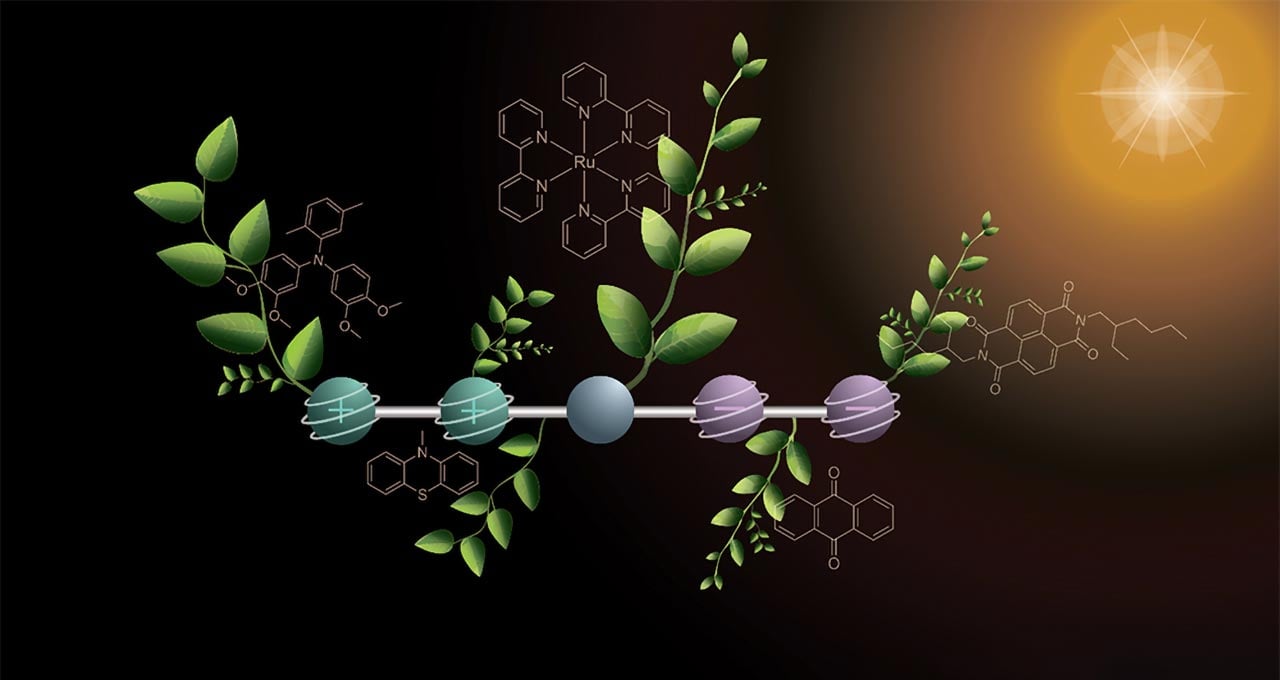
In Nature Chemistry, Professor Oliver Wenger and doctoral student Mathis Brändlin describe an important advance toward this goal of artificial photosynthesis. They have engineered a specially designed molecule that, when exposed to light, can hold four charges at the same time – two positive and two negative.
Being able to temporarily store several charges is a crucial step in turning sunlight into usable chemical energy. Those charges can then be applied to trigger reactions, such as splitting water into hydrogen and oxygen.
The molecule itself is built from five linked components, each with a distinct role. On one end, two parts release electrons and in doing so become positively charged. At the opposite end, two other parts absorb those electrons and turn negatively charged. At the center, the researchers placed a light-sensitive unit that captures solar energy and initiates the electron transfer.
Two Flashes of Light, Four Charges
In order to generate the four charges, the researchers took a stepwise approach using two flashes of light. The first flash of light hits the molecule and triggers a reaction in which a positive and a negative charge are generated. These charges travel outward to the opposite ends of the molecule. With the second flash of light, the same reaction occurs again, so that the molecule then contains two positive and two negative charges.
Works Even in Dim Light
“This stepwise excitation makes it possible to use significantly dimmer light. As a result, we are already moving close to the intensity of sunlight,” explains Brändlin. Earlier research required extremely strong laser light, which was a far cry from the vision of artificial photosynthesis. “In addition, the charges in the molecule remain stable long enough to be used for further chemical reactions.”
That being said, the new molecule has not yet created a functioning artificial photosynthesis system. “But we have identified and implemented an important piece of the puzzle,” says Oliver Wenger. The new findings from the study help to improve our understanding of the electron transfers that are central to artificial photosynthesis. “We hope that this will help us contribute to new prospects for a sustainable energy future,” says Wenger.
Reference: “Photoinduced double charge accumulation in a molecular compound” by Mathis Brändlin, Björn Pfund and Oliver S. Wenger, 25 August 2025, Nature Chemistry.
DOI: 10.1038/s41557-025-01912-x
Never miss a breakthrough: Join the SciTechDaily newsletter.
Source link

