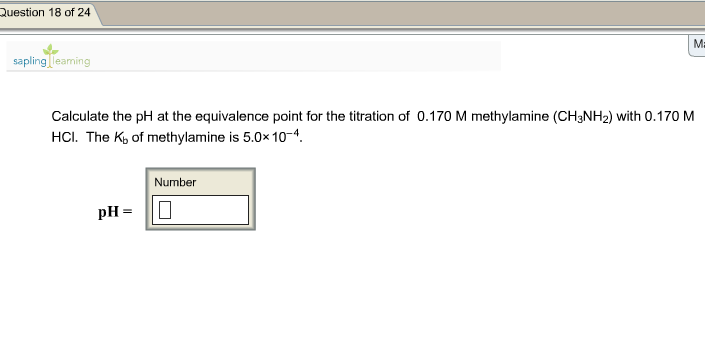
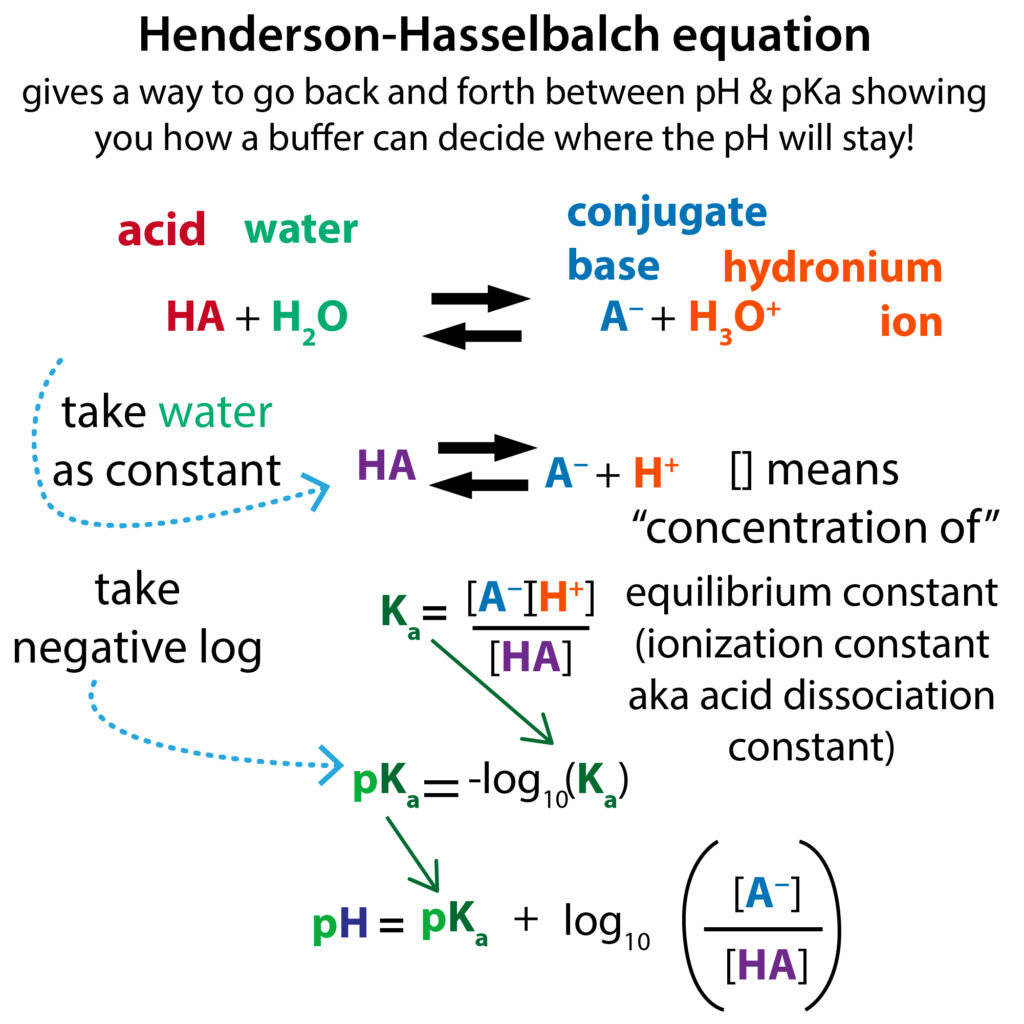
Buffer calculation: Tris buffer - Tris(hydroxymethyl)-aminomethane Calculate the pH of a buffer made from 50 mL of 0.10M tris a
STOCK SOLUTION RECIPIES: Tris-HCl Buffer 10X Tris-HCl (0.5M Tris Base, pH7.6): Trizma Base ---------------------------------- 6
![PDF] Calculation of the pH of Buffer Solution of 2-[ N -Morpholino]ethanesulfonic Acid (MES) from 5°C to 55°C | Semantic Scholar PDF] Calculation of the pH of Buffer Solution of 2-[ N -Morpholino]ethanesulfonic Acid (MES) from 5°C to 55°C | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4f93c0cf01a6aa114c33424b0d97a3f0e6b3da18/3-Table1-1.png)
PDF] Calculation of the pH of Buffer Solution of 2-[ N -Morpholino]ethanesulfonic Acid (MES) from 5°C to 55°C | Semantic Scholar
![PDF] Calculation of the pH of Buffer Solution of 2-[ N -Morpholino]ethanesulfonic Acid (MES) from 5°C to 55°C | Semantic Scholar PDF] Calculation of the pH of Buffer Solution of 2-[ N -Morpholino]ethanesulfonic Acid (MES) from 5°C to 55°C | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4f93c0cf01a6aa114c33424b0d97a3f0e6b3da18/4-Table4-1.png)
PDF] Calculation of the pH of Buffer Solution of 2-[ N -Morpholino]ethanesulfonic Acid (MES) from 5°C to 55°C | Semantic Scholar




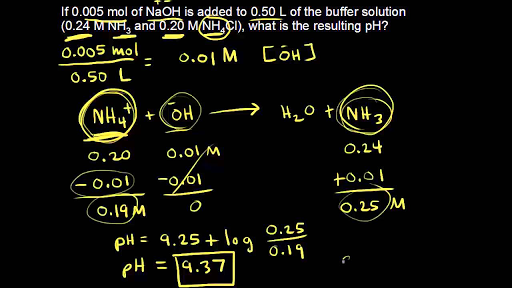








![Tris Hydrochloride [C4H11NO3.HCl] Molecular Weight Calculation - Laboratory Notes Tris Hydrochloride [C4H11NO3.HCl] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/11/tris-hydrochloride-molecular-weight-calculation-300x225.jpg)