Vaccinated but Still Got COVID? A New Study Helps Explains Why

A Japanese study has found that one group of people lose vaccine protection more quickly than others, even though they begin with higher antibody levels.
Two healthcare workers receive COVID-19 vaccines on the same day. Both develop strong antibody responses at first, but six months later, one remains protected while the other becomes infected. A new study in Science Translational Medicine may explain why this occurs.
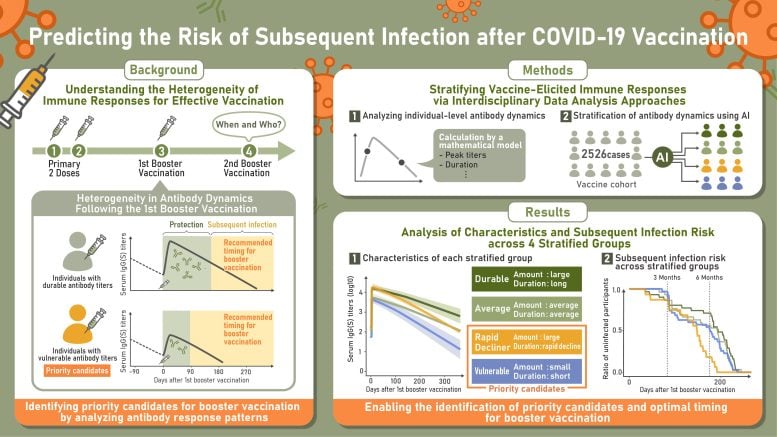
The researchers monitored antibody responses after vaccination and identified four distinct patterns following the first booster. Those with the highest initial antibody levels but the steepest decline were more likely to get infected sooner. Individuals with lower concentrations of IgA(S) antibodies, which help defend the nose and throat, also faced a greater risk. These findings indicate that tracking how antibodies change over time could help pinpoint people who are more susceptible to infection.
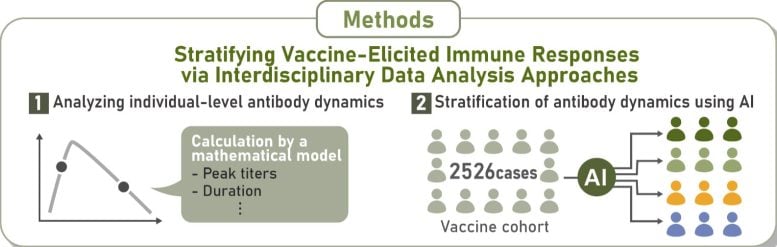
The study, led by scientists at Nagoya University in Japan, followed 2,526 participants over an 18-month period to examine immune responses from the first vaccine dose through subsequent boosters. Using long-term data and AI-driven analysis, the team built a mathematical system to classify immune responses, making them the first to define and characterize a group they called “rapid-decliners.”
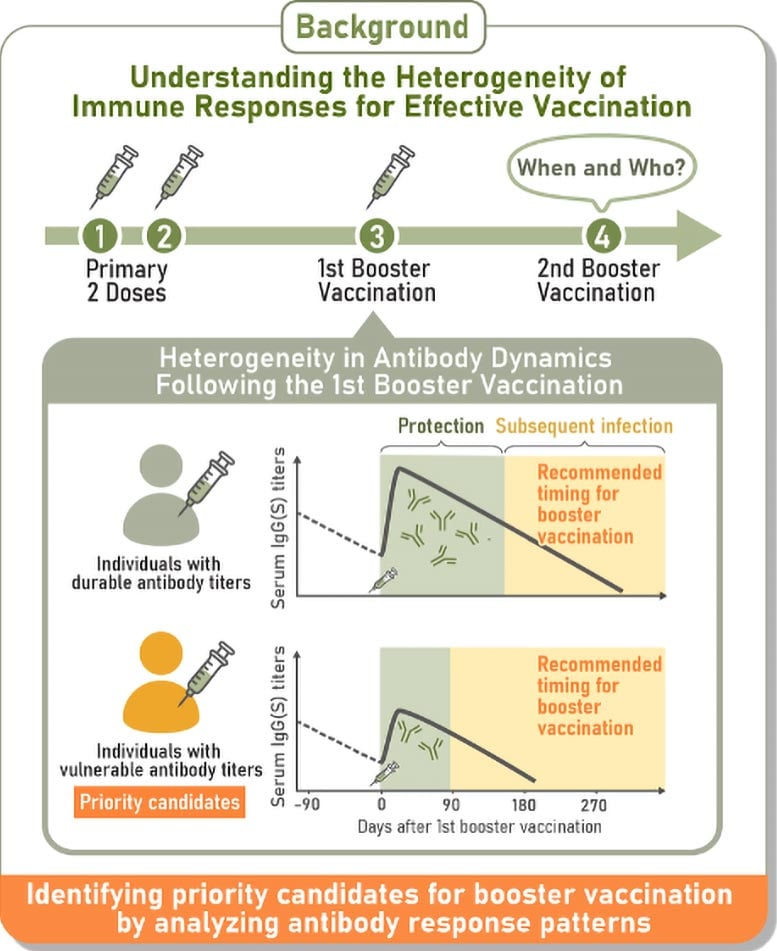
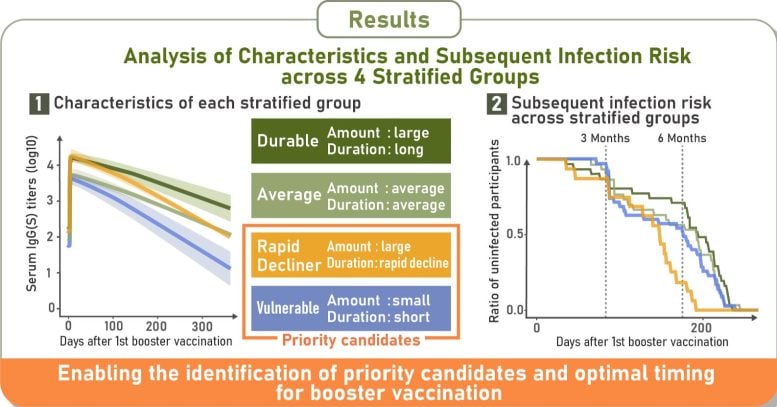
The results showed four consistent immune response types: durable responders, who maintained high antibody levels; rapid-decliners, who began with strong responses but lost them quickly; vulnerable responders, who generated low antibody levels that declined rapidly; and intermediate responders, who fell between these groups.
Immunity that peaks early and then drops
Shingo Iwami, senior author and professor at Nagoya University’s Graduate School of Science, noted that the results for the rapid-decliner group were unexpected. “In spite of their impressive initial immune response, they caught COVID-19 sooner than other groups, while durable responders maintained protection for longer periods. One-time blood tests for IgG antibodies, the antibody type we used for classification, couldn’t detect this risk. Only by tracking changes over months did we see the pattern,” he explained.
A breakthrough or subsequent infection occurs when someone becomes infected after vaccination because the virus bypasses the immunity provided by the vaccine. The researchers discovered that individuals whose antibodies dropped more quickly, whether due to initially low levels or rapid decline (vulnerable responders and rapid-decliners), faced a slightly higher risk of earlier breakthrough infections.
Following booster vaccinations, 29% of participants were classified as durable responders, 28% as vulnerable responders, and 19% as rapid-decliners. The rest showed intermediate responses. Breakthrough infection rates were fairly close between groups, with 5.2% of durable responders and 6% of vulnerable responders, and rapid-decliners affected.
Breakthrough infections linked to IgA(S) antibody levels
The study also revealed that participants who experienced breakthrough infections had lower levels of IgA(S) antibodies in their blood several weeks after vaccination. These antibodies protect the nose and throat and are our first line of defense against respiratory viruses.
Importantly, the researchers found a strong correlation between blood IgA(S) levels and nasal IgA(S) levels, suggesting that blood tests can reliably indicate the strength of immune protection in airways. As a result, measuring blood IgA(S) levels after vaccination may help identify individuals at higher risk for breakthrough infection, especially among vulnerable groups.
While these results provide a foundation for future research, Professor Iwami emphasized the importance of identifying the underlying biological mechanisms responsible for the rapid decline in antibody levels in order to develop more effective vaccination strategies. Previous research points to factors such as age, genetic variation, vaccine-specific characteristics, and environmental influences, including sleep habits, stress levels, and medications being taken at the same time.
“This is the first time we’ve been able to clearly group how people respond to COVID-19 vaccines,” Professor Iwami noted. “Identifying the rapid-decliner pattern is especially important—it helps explain why some people may need boosters sooner than others. This could potentially contribute to better, more personalized vaccination strategies. However, whether antibody testing can be used widely depends on cost, accuracy, and if the benefits are worthwhile compared to current strategies. More research is needed to understand its full potential.”
Reference: “Longitudinal antibody titers measured after COVID-19 mRNA vaccination can identify individuals at risk for subsequent infection” by Hyeongki Park, Naotoshi Nakamura, Sho Miyamoto, Yoshitaka Sato, Kwang Su Kim, Kosaku Kitagawa, Yurie Kobashi, Yuta Tani, Yuzo Shimazu, Tianchen Zhao, Yoshitaka Nishikawa, Fumiya Omata, Moe Kawashima, Toshiki Abe, Yoshika Saito, Saori Nonaka, Morihito Takita, Chika Yamamoto, Hiroshi Morioka, Katsuhiro Kato, Ken Sagou, Tetsuya Yagi, Takeshi Kawamura, Akira Sugiyama, Aya Nakayama, Yudai Kaneko, Risa Yokokawa Shibata, Kazuyuki Aihara, Tatsuhiko Kodama, Akifumi Kamiyama, Tomokazu Tamura, Takasuke Fukuhara, Kenji Shibuya, Tadaki Suzuki, Shingo Iwami and Masaharu Tsubokura, 17 September 2025, Science Translational Medicine.
DOI: 10.1126/scitranslmed.adv4214
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google, Discover, and News.
Source link

