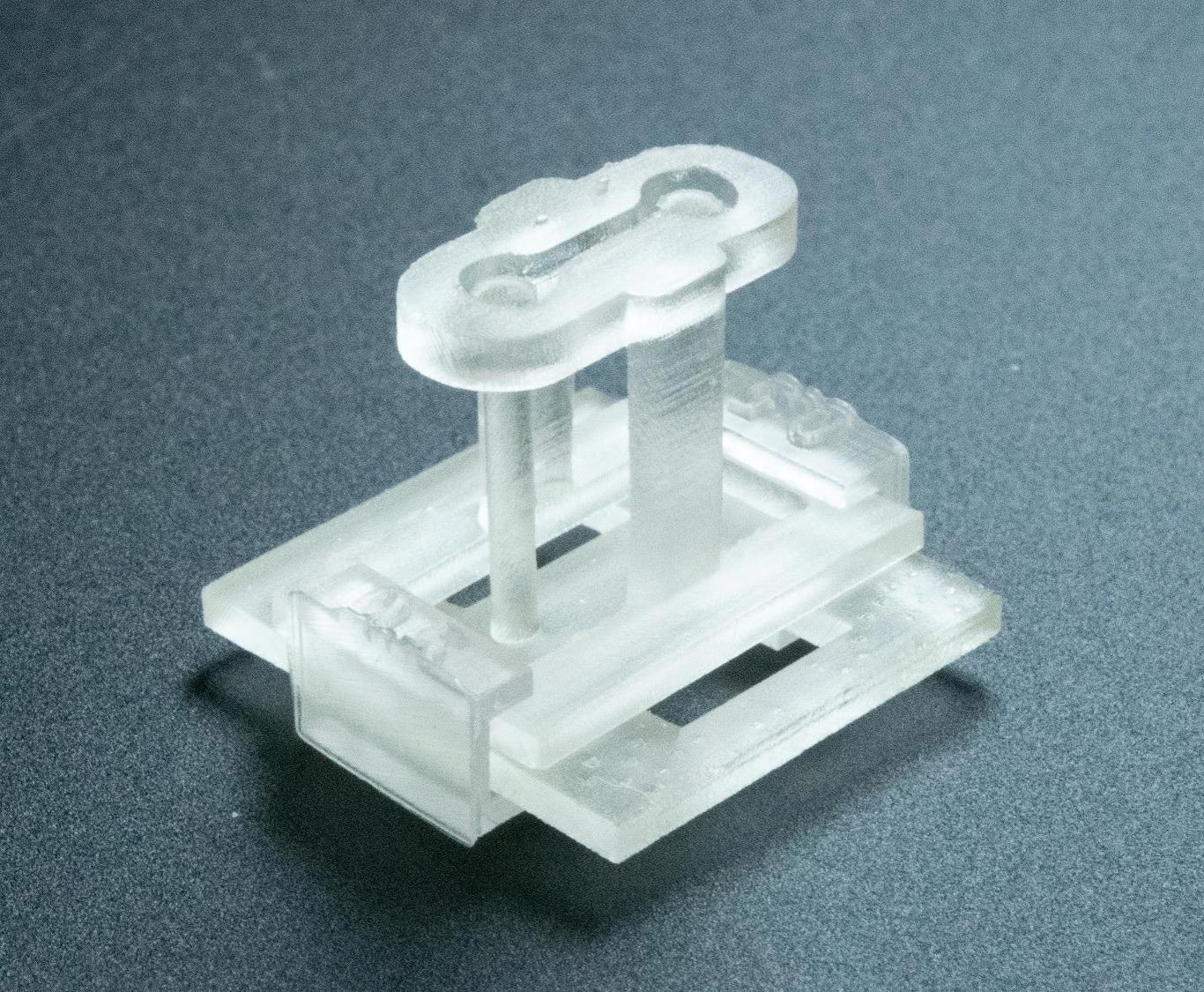
The device is compact enough to rest on a fingertip and is compatible with current tissue-engineering technology.
A newly developed 3D-printed device offers scientists the ability to build human tissue models with far greater precision and complexity. The tool, created by an interdisciplinary team at the University of Washington and UW Medicine, is designed to integrate easily into existing laboratory methods.
Recent progress in 3D tissue engineering has already improved the speed and accuracy of building cell-based systems, giving biomedical researchers powerful new ways to design and test treatments for a wide range of diseases. A central aim of the field is to recreate laboratory environments that mimic the natural conditions cells experience inside the body.
One common method involves suspending cells within a gel positioned between two freestanding posts. This setup has been used to grow tissues such as heart, lung, skin, and muscle. While effective at allowing cells to function in a life-like way, the approach has limitations: it is difficult to examine how multiple tissue types interact. Achieving finer control over the composition and arrangement of cells could make it possible to model complex conditions, including neuromuscular disorders.
STOMP enables patterned tissue environments
A paper published in Advanced Science details how the new platform lets scientists examine how cells respond to mechanical and physical cues, while creating distinct regions in a suspended tissue. The 3D-printed device is known as STOMP (Suspended Tissue Open Microfluidic Patterning).
Ashleigh Theberge, UW professor of chemistry, and Nate Sniadecki, professor of mechanical engineering and interim codirector of the UW Medicine Institute for Stem Cell and Regenerative Medicine, led the scientific team. The group showed that their device can recreate biological interfaces like bone and ligament, or fibrotic and healthy heart tissue.
The first authors of the paper were Amanda Haack, a student in the School of Medicine’s medical scientist program and postdoctoral fellow in the Theberge Lab, and Lauren Brown, a Ph.D. student in chemistry. UW faculty members Cole DeForest, professor of chemical engineering and bioengineering, and Tracy Popowics, professor of oral biology in the School of Dentistry, are coauthors.
Casting tissues with microfluidic precision
STOMP builds on a tissue-engineering technique known as casting, which the researchers describe through the simple analogy of making Jell-O in a mold. In laboratory practice, the “gel” is a mixture of living and synthetic materials, placed into a supporting frame with a pipette rather than poured. STOMP takes this approach further by using capillary action—similar to the way water rises in a straw—to allow scientists to precisely position different cell types in chosen patterns, much like arranging fruit pieces evenly within Jell-O.
To evaluate its potential, the team tested STOMP in two experiments: one examined the contractile behavior of engineered heart tissue in both diseased and healthy states, while the other recreated the ligament that secures a tooth within its bone socket.
The device itself is only the size of a fingertip. It attaches to a two-post platform originally designed by the Sniadecki Lab for measuring the contractile forces of heart cells. Within this small structure lies an open microfluidic channel equipped with geometric features that control both the spacing and arrangement of cell types, enabling the creation of distinct regions in suspended tissue—all without requiring extra tools or added complexity.
Design features improve tissue versatility
Hydrogel technology from the DeForest Research Group souped up STOMP with another design feature: degradable walls. Tissue engineers can break down the sides of the device and leave the tissues intact.
“Normally when you put cells in a 3D gel,” Sniadecki said, “they will use their own contractile forces to pull everything together — which causes the tissue to shrink away from the walls of the mold. But not every cell is super strong, and not every biomaterial can get remodeled like that. So that kind of nonstick quality gave us more versatility.”
Theberge is excited about how other teams will use STOMP.
“This method opens new possibilities for tissue engineering and cell signaling research,” she said. “It was a true team effort of multiple groups working across disciplines.”
Reference: “Suspended Tissue Open Microfluidic Patterning (STOMP)” by Amanda J. Haack, Lauren G. Brown, Alex J. Goldstein, Priti Mulimani, Jean Berthier, Asha R. Viswanathan, Irina Kopyeva, Jamison M. Whitten, Ariel Lin, Serena H. Nguyen, Thomas P. Leahy, Ella E. Bouker, Ruby M. Padgett, Natalie A. Mazzawi, Jodie C. Tokihiro, Ross C. Bretherton, Aaliyah Wu, Stephen J. Tapscott, Cole A. DeForest, Tracy E. Popowics, Erwin Berthier, Nathan J. Sniadecki and Ashleigh B. Theberge, 29 April 2025, Advanced Science.
DOI: 10.1002/advs.202501148
The National Institutes of Health (NIH) supported the research through R35GM128648, R35GM138036, R01HL149734, R03DE029827, T32CA080416, F30HL158030, R90DE023059, 5TL1TR002318-08, and a supplement to R35GM128648. This work was also partially supported by the UW, Friends of FSH Research, The Chris Carrino Foundation for FSHD and fellowship funds from Senator Paul D. Wellstone Muscular Dystrophy Specialized Research Center – Seattle (NIAMS P50AR065139), and a gift from Ionis Pharmaceuticals.
Never miss a breakthrough: Join the SciTechDaily newsletter.
Source link